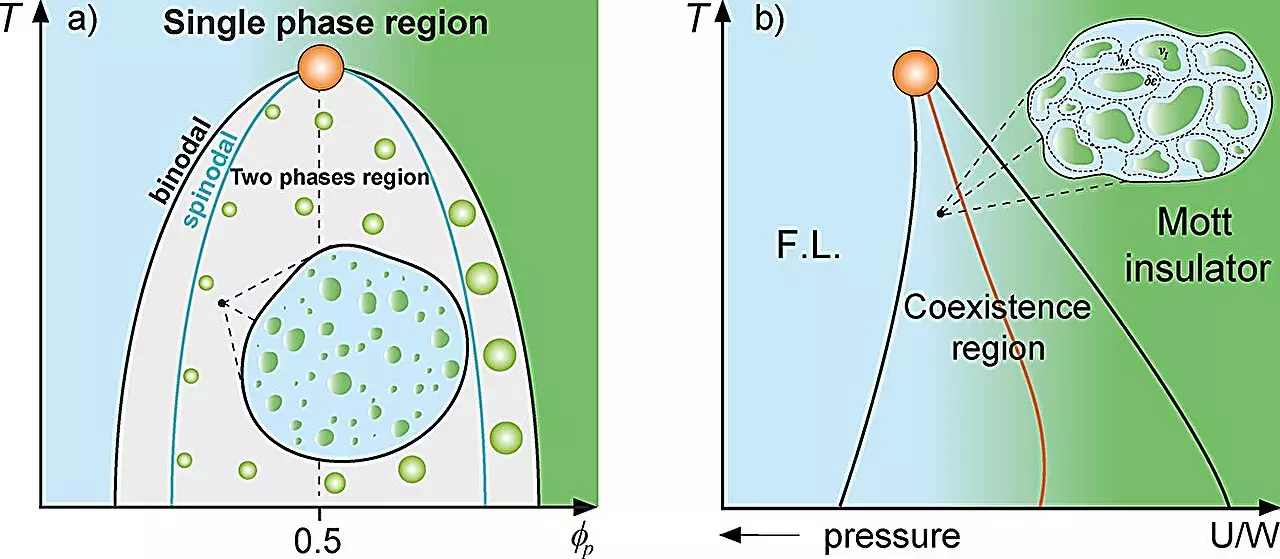
In the realm of biological sciences, the study of protein interactions and dynamics has unveiled intricate mechanisms that govern cellular functions. Drawing parallels from condensed matter physics, researchers have utilized classical mixture theory to better understand how proteins compartmentalize within cells. This innovative approach posits that just as various phases coexist within a physical system—like supercooled water or metal puddles in an insulating matrix—biological systems can similarly exhibit phase separation, leading to the formation of protein-rich droplets. Such findings illuminate the significance of environmental conditions and thermodynamic principles in cellular behavior, potentially reshaping our understanding of cellular organization and functionality.
A groundbreaking study from São Paulo State University (UNESP) introduces a compelling analogy between cellular dynamics and the Griffiths phase observed in magnetic systems. The research, led by Professor Mariano de Souza and Ph.D. candidate Lucas Squillante, draws a vivid comparison between the emergence of ‘rare regions’ in magnetic matrices and the behavior of proteins under certain intracellular conditions. In magnetic materials, these rare regions can significantly slow down the dynamics of the system, contributing to a richer understanding of phase transitions. Similarly, the study highlights how protein droplet formation within a cell leads to reduced dynamics and might be conceptualized as a Griffiths-like cellular phase.
This phase serves to explain how proteins, when produced in high quantities, undergo liquid-liquid phase separation, influencing their interaction and transport mechanisms. Understanding this phenomenon is crucial for unraveling complex biological processes and how they may relate to broader evolutionary mechanisms.
Utilizing thermodynamic principles such as the Grüneisen parameter, the Flory-Huggins model, and the Avramov-Casalini model, the researchers reveal that cellular dynamics experience a significant slowdown near the binodal line—the threshold for phase separation. This finding sheds light on the intricate balance of forces at play within the cell, illustrating how concentration changes can promote or inhibit the assemblage of proteins into droplets. Such compartmentalization is vital for maintaining cellular order and function, providing a framework for understanding the foundational aspects of life itself.
The model presented in the study connects to Aleksandr Oparin’s theory regarding the origin of life, suggesting that the primordial conditions conducive to life’s emergence may hinge on such dynamic processes. As life evolved, the significance of slow-moving coacervates likely enabled the stability and diversity required for survival. This correlation not only emphasizes the historical importance of protein dynamics but also opens avenues for future research on cellular evolution.
The study delves deeper into biological intricacies by discussing the critical role of chirality—an essential property of molecular structures. The concept of homochirality, wherein one type of chiral molecule predominates, is essential for proper biological function. The researchers found that increased diffusion time of proteins is associated with decreased stochastic fluctuations, which in turn optimizes gene expression. This finding reinforces the idea that not only spatial organization but also the inherent properties of the molecules involved are fundamental to cellular operations.
The implications of chirality extend to understanding disease processes as well. For instance, alterations in chiral properties may disrupt cellular functions, potentially leading to pathological outcomes. This intersection of molecular dynamics and disease manifestation underscores the importance of interdisciplinary approaches in research.
Beyond basic science, the findings of this study carry significant implications for practical applications, particularly in the context of disease. The researchers emphasize that liquid-liquid phase separation is a pivotal factor in various diseases, including cancers and neurodegenerative disorders. The ability of protein droplets to compartmentalize molecular interactions can influence cell mutation and disease progression, warranting further exploration into therapeutic strategies.
As highlighted by co-author Professor Marcos Minicucci, understanding how phase separation operates could lead to novel interventions in disease management. The variability in how liquid-liquid phase separation impacts different diseases presents both challenges and opportunities for targeted therapies. For example, recent studies have pointed out connections between protein compartmentalization and conditions like cataracts, illustrating its extensive influence on health.
The research elucidated by the UNESP team signifies a pivotal moment in the cross-disciplinary collaboration between physics and biology. By drawing on concepts from condensed matter physics, we can acquire novel insights into the complexity of cellular dynamics. Understanding protein compartmentalization not only enhances our comprehension of fundamental biological processes but also paves the way for innovative treatment strategies for various diseases. As our grasp of these intricate mechanisms deepens, the potential to harness this knowledge in medical applications continues to expand, ultimately improving our approach to health and disease management.